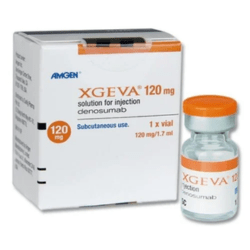
What is the active substance in Xgeva?
Denosumab is the active substance in Xgeva.
How is Denosumab supplied?
This therapeutic drug is supplied for Injection: 120 mg/1.7 mL (70 mg/mL) solution in a single-dose vial for subcutaneous use only.
Who has introduced the generic version of Denosumab in India?
Dr. Reddy Laboratories Ltd has introduced a generic version of Denosumab in India under the brand Xgeva.
How much does Denosumab cost?
Please Call/WhatsApp: +91 8130290915 or dial our TOLL-FREE Number: 1800-889-1064 to know the exact Denosumab cost in India. If you want to buy Denosumab online, then THE INDIAN PHARMA (TIP) can help you access this medicinal product at the most competitive price through legal channels.
Is Denosumab available in India?
Denosumab is a pharmaceutical prescription medicinal product that can only be dispensed against a valid prescription from a treating physician. THE INDIAN PHARMA (TIP) can facilitate the supply of this drug to all locations after fulfilling the legal requirements (if applicable). Please contact us at +91 8130290915 | TOLL-FREE: 1800-889-1064 or at info@theindianpharma.com to buy Denosumab from India at the best price.
Can I get Denosumab injections even if I am not based in India?
THE INDIAN PHARMA (TIP) helps patients access Denosumab, which is not approved in their home country, against a valid prescription and in conformity with their respective country’s local laws and regulations.
Send your inquiry to find generic Denosumab in UAE, Iraq, Iran, Argentina, Brazil, Chile, Colombia, Peru, Saudi Arabia, Jordan, Mexico, Venezuela, Romania, Switzerland, Georgia, Turkey, Italy, UK, Ukraine, Sri Lanka, Afghanistan, Poland, Slovakia, Armenia, Kazakhstan, Germany, Azerbaijan, Latvia, Moldova, the Philippines, Tajikistan, Turkmenistan, Uzbekistan, Mongolia, Algeria, Mauritius, Uganda, Zimbabwe, Australia, New Zealand, China, Cambodia, Indonesia, Malaysia, Singapore, Thailand, Vietnam, Kenya, Morocco, Hong Kong.
Where can I get the best Denosumab Cost Price in India?
The price of Denosumab is low and reasonable in India compared to other countries. If you wish to buy Denosumab injections online from India at an affordable price, contact us today. We will help facilitate the supply of this therapeutic drug at the lowest possible prices.
Is it safe to buy Denosumab online from India?
Yes, one can buy generic Denosumab online at the best price from THE INDIAN PHARMA (TIP) if this drug is not registered or is unavailable in their country. TIP can help facilitate the supply/export of Denosumab injections through legal channels.