Description
Taffic Uses:
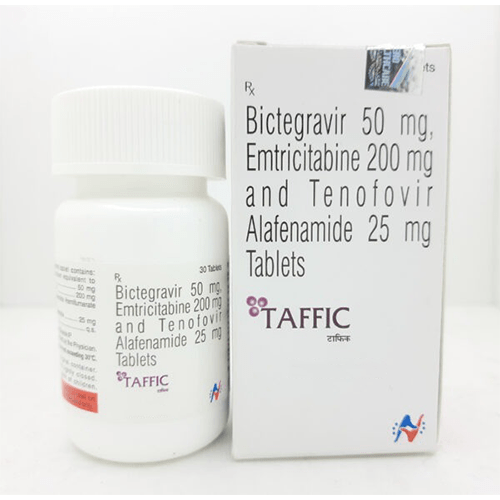
Taffic is a complete HIV-1 treatment that combines 3 powerful medicines (Bictegravir/Emtricitabine/Tenofovir Alafenamide) into 1 small pill. Taffic is used for adults and children with HIV-1 who weigh at least 55 lbs and are either:- new to HIV-1 treatment OR
- replacing their current regimen
Dosage:
The recommended dosage in adults and pediatric patients weighing at least 25 kg is One Tablet containing Bictegravir 50mg/ Emtricitabine 200mg/ Tenofovir Alafenamide 25mg taken orally once daily with or without food. Dosage of Taffic is taken on a regular dosing schedule. Do not miss any doses as it can result in the development of resistance.
Side Effects:
The most commonly reported side effects due to taffic tablets include:
- diarrhea
- nausea
- headache
Warnings and Precautions:
- Prior to or when initiating Taffic test patients for hepatitis B virus infection. Prior to or when initiating this therapy, and during therapy, assess serum creatinine, urine glucose, estimated creatinine clearance and urine protein. In patients with chronic kidney complications, also evaluate serum phosphorus.
- Bictegravir, Emtricitabine, Tenofovir Alafenamide is not recommended in patients with estimated creatinine clearance of 15 to below 30 mL/min, or below 15 mL/min who are not with chronic hemodialysis, or below 15 mL/min who have no history of antiretroviral treatment.
- Taffic is contraindicated to be used with dofetilide due to the risk for increased dofetilide plasma concentrations and associated serious or life-threatening events. Do not use Taffic with rifampin because of decreased BIC plasma concentrations, which may result in the loss of therapeutic effect and development of resistance to Taffic.
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, may occur with the use of nucleoside analogs, including emtricitabine, a component of Taffic, and tenofovir DF, another prodrug of tenofovir, alone or along with other antiretrovirals.
- Suspend treatment with Bictegravir/Emtricitabine/Tenofovir Alafenamide in any patient who develops clinical or laboratory findings suggestive of pronounced hepatotoxicity or lactic acidosis.
- Patients should inform their healthcare provider promptly of any signs/symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs/symptoms of inflammation from previous infections may emerge soon after anti-HIV therapy is started.
- Patients are informed that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to Taffic. Women with HIV-1 infection should avoid breastfeeding because HIV-1 may be passed to the baby in breast milk.