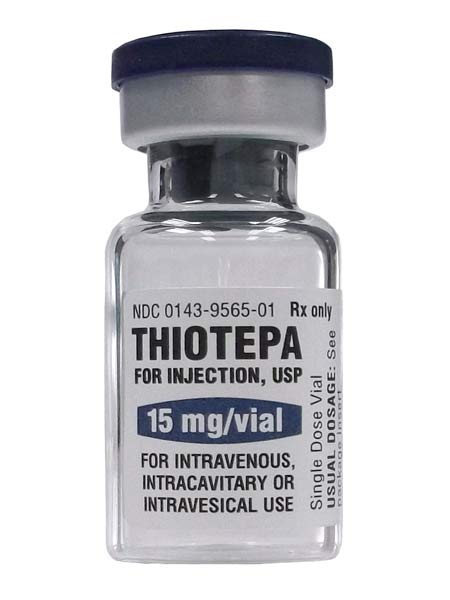
Description
(TEPADINA is registered trademarks ADIENNE SA)
Tepadina – Thiotepa 15 mg and 100 mg is supplied for Tenders, Emergency imports, Orphan drug, Reference listed drugs, Comparator Drug, Name patient medicine and Innovator samples etc.
What is TEPADINA:
TEPADINA is a medicine that contains the active substance Thiotepa and is available as a Single Vial.
In how many countries TEPADINA is approved for marketing?
TEPADINA is available/approved in USA and Europe. We can help you to access it in India.
What is TEPADINA used for?
Tepadina is indicated for the treatment of-
- adenocarcinoma of the breast or ovary.
- for controlling intracavitary effusions secondary to diffuse or localized neoplastic diseases of various serosal cavities.
- For treatment of superficial papillary carcinoma of the urinary bladder.
How can I get TEPADINA in India?
TEPADINA is a prescription only drug and is not approved for marketing in India.
The Indian Pharma (IP) is a consulting pharmaceutical company which assists Patients in accessing medicines in India. The Indian Pharma facilitates such access only against valid prescriptions in conformity with all local laws and regulations.
Patients/ Clinicians / Researchers can contact IP at +91-120-4080562 or write to at – info@theindianpharma.com
We facilitate by-
- Helping in documentation to import the medicine for personal use
- Finding Genuine and reliable source in USA, Europe and Japan
- Ensuring 100% transparency.
TEPADINA can be shipped to Mumbai, Kolkata, Hyderabad, Chennai, Ahmedabad, Delhi, Bangalore, Jaipur, Lucknow, Cochin and Pune and other cities in India.
The Indian Pharma can facilitate the supply of TEPADINA (prescription medicines) to all locations in the world and in India after fulfilling the legal requirement.
The Indian Pharma does not manufacture, supply, re-sell or retail any drugs or medicines. All trademarks and other intellectual property in relation to drugs and medicines supplied to you by suppliers are owned by their respective manufacturers or licensees
Can I get TEPADINA even if I am not based in India?
Almost all countries across the world have provisions for granting access to drugs prior to marketing approval for personal use for patients who have exhausted all other treatment options available in their country
The Indian Pharma (IP) can help patients in accessing/importing TEPADINA, unapproved in their home country against a legitimate prescription and in conformity with all local laws and regulations of their home country.
Send your enquiry to find-
- TEPADINA in South East Asia – China (Beijing, Chongqing, Shanghai, Tianjin and Shenzhen), Cambodia, Indonesia, Malaysia, the Philippines, Singapore, Thailand, Vietnam, Hong Kong.
- TEPADINA in United Arab Emirates – Iraq, Iran, Saudi Arabia, Jordan.
- TEPADINA in North America – Mexico.
- TEPADINA in South America – Argentina, Brazil, Chile, Colombia, Peru, Venezuela.
- TEPADINA in Europe – Romania, Switzerland, Georgia, Turkey, Italy, UK, Ukraine, Azerbaijan, Latvia, Poland, Slovakia.
- TEPADINA in Russia CIS – Armenia, Kazakhstan, Moldova, Tajikistan, Turkmenistan, Uzbekistan, Mongolia.
- TEPADINA in African Countries – Algeria, Mauritius, Uganda, Zimbabwe.
- TEPADINA in Australia and New Zealand.