What does ATGAM contain as an active substance?
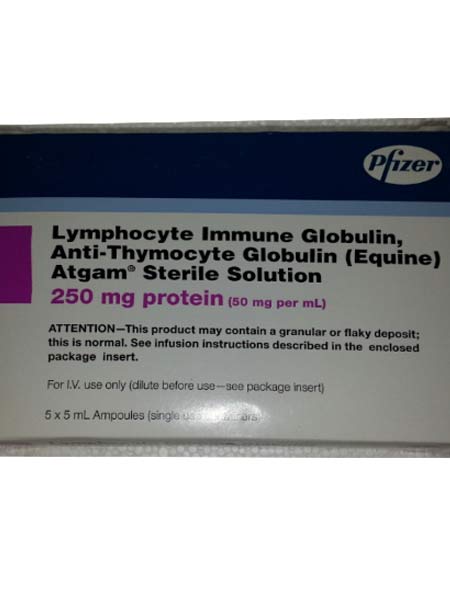
ATGAM specifically contains the active substance Anti-Thymocyte Globulin-Equine.
How is ATGAM supplied?
ATGAM is supplied as 50 mg/mL concentrate for solution for infusion for intravenous use. It is available by prescription only.
How Should I Store ATGAM?
Store this therapeutic drug in a refrigerator at 2°F to 8°C (36°F to 46°F). Avoid freezing it. Store in the original carton to protect from light.
Is the ATGAM Injection Available In India?
ATGAM is a (prescription medicine, doctor-prescribed medication, or health care professional-prescribed drug) pharmaceutical drug that can be legally dispensed against a medical prescription. THE INDIAN PHARMA (TIP) can facilitate the supply of ATGAM after fulfilling the legal requirement (if applicable). Please contact us via
+91 8130290915 or TOLL-FREE Number: 1800-889-1064 or write to us at info@theindianpharma.com to buy ATGAM online in India.
Where can I Access ATGAM (Anti-Thymocyte Globulin-Equine) at the best price in India?
THE INDIAN PHARMA (TIP), a certified pharmaceutical facilitator/supplier/importer/exporter and WHO-GDP certified offer ATGAM in India. The FDA-approved Anti-Thymocyte Globulin-Equine (brand name ATGAM) is now registered in India, and Indian patients can purchase it at the lowest price. To confirm an order for this therapeutic drug, a valid doctor’s prescription must be submitted.
How much does ATGAM (Anti-Thymocyte Globulin-Equine) cost in India?
Prices may fluctuate over time due to market dynamics and regulatory changes. To obtain accurate and up-to-date information on
ATGAM/ATG 50 mg/mL cost in India, it is recommended to Call/WhatsApp +91 8130290915 or send mail to info@theindianpharma.com.
Can I get ATGAM/ATG even if I am not based in India?
THE INDIAN PHARMA (TIP) helps patients and healthcare professionals access ATGAM (Anti-Thymocyte Globulin-Equine), not approved in their home country against the valid prescription and in conformity with the local laws and regulations of their respective country.
Where can I get ATGAM (Anti-Thymocyte Globulin-Equine) at the best price?
To get the best
ATGAM injection price in India and other countries, always buy this medication from a Pharmaceutical Wholesaler Company that is GDP-certified. Noida-based THE INDIAN PHARMA (TIP) can offer the best price for ATGAM (Anti-Thymocyte Globulin-Equine).
What is the procedure for buying ATGAM (Anti-Thymocyte Globulin-Equine) from India?
Patients can simply fill out the order form or send mail to
info@theindianpharma.com. Patients can also send WhatsApp messages to +91 8130290915 or dial the TOLL-FREE Number: 18008891064. We will reply ASAP with the details of the ATGAM injection price and the procurement procedure.
Note:- The order will be confirmed only after the valid receipt of the prescription from the Physician.
Is it safe to buy ATGAM (Anti-Thymocyte Globulin-Equine) online in India?
Yes, one can
buy ATGAM injection online from India-based THE INDIAN PHARMA (TIP) if this therapeutic drug is not registered or is unavailable in their country. We can facilitate the supply of ATGAM injection through legal channels.
Can I get ATGAM injection even if I am not based in India?
THE INDIAN PHARMA (TIP) helps patients access generic ATGAM , which is not approved in their home country against a valid prescription and is in conformity with their respective country’s local laws and regulations. Send your inquiry to find generic ATGAM in UKRAINE , Saudi Arabia , Jordan , Mexico , UZBEKISTAN , Brazil , Chile , Colombia , Peru , Venezuela , Romania , Switzerland , Georgia , Turkey , Italy , Sri Lanka , Afghanistan , Germany , Azerbaijan , Latvia , Poland , Armenia , Kazakhstan , Moldova , Tajikistan , Mongolia , Algeria , Mauritius , Uganda , Zimbabwe , Australia , New Zealand , China , Cambodia , Indonesia , Malaysia , Philippines , Singapore , Thailand , Vietnam , Hong Kong , UAE , Iran , Argentina , UK , Slovakia , Turkmenistan , TRINIDAD & TOBAGO , LIBERIA , FRANCE , SLOVAK REPUBLIC , EGYPT , GUINEA , KENYA , BENIN , DOMINICAN REPULIC , NEPAL , ETHIOPIA , SOMAALIA , UNITED ARAB EMIRATES , CAMEROON , ST LUCIA , MALI , TANZANIA , CONGO, THE DEMOCRATIC REPUBLIC OF THE , COTE D IVOIRE , CAYMAN ISLANDS , NIGERIA , ZAMBIA , QATAR , MADAGASCAR , GAUTEMALA , OMAN , MOROCCO , HUNGARY , MACEDONIA,THE FORMER YUGOSLAV REPUBLIC OF , NAMBIA , UNITED STATES , JAMAICA , CONGO , SERBIA , MYANMAR , SOUTH AFRICA , SUDAN , ECUADOR , SOMALIA , FIJI , MALAWI , PANAMA , NICARAGUA , BELIZE , GREECE , BOSNIA & HERZEGOVINA , TAIWAN , SENEGAL , BURKINA FASO , PAPUA NEW GUINEA , GHANA , PARAGUAY , BANGLADESH , MALTA , MALDIVES , NIGER , SIERRA LEONA , LEBANON , BARBADOS , TOGO , PAKISTAN , ST KITTS-NEVIS-ANGUILLA , ERITREA , GABON , MOZAMBIQUE , GRENADA , EL SALVADOR , RUSSIA , GUYANA , BRITISH VIRGIN ISLANDS , LITHUANIA , BOTSWANA , HAITI , SAINT VINCENT AND GRENADINES , NETHERLANDS , GUATEMALA , ANGOLA , LAO , KYRGHYSTAN , YEMEN , DOMINICA , CONGO D REPULIC , CANADA , BURUNDI , KAZAKISTAN , BELGIUM , MOLDOVA,REPUBLIC OF , LAO PEOPLE’S DEMOCRATIC REPUBLIC , SAINT KITTS AND NEVIS , SAINT LUCIA , SEYCHELLES , RWANDA , LIBYA , IRELAND , ST VINCENT , SPAIN , BOLIVIA , NETHERLANDS ANTILLES , SURINAME , JAPAN , NAMIBIA , ALBANIA , MAURITANIA , BAHAMAS , DOMINICAN REPUBLIC , BULGARIA , PORTUGAL , YEMEN , KOREA , COSTA RICA , TUNISIA , GAMBIA.