Description
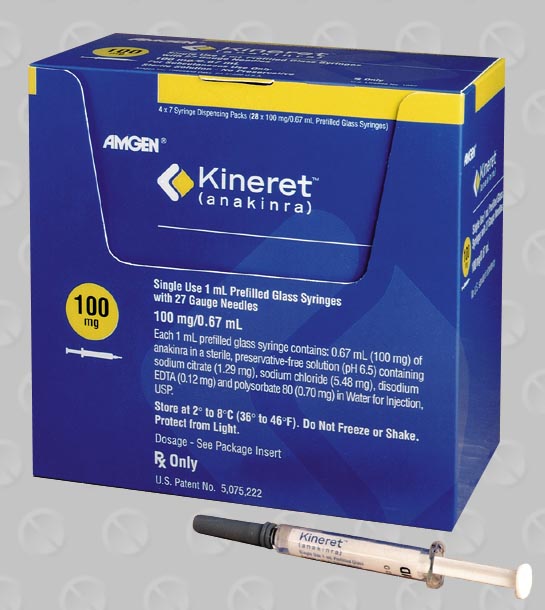
Kineret (Anakinra Injection) Uses
Anakinra is an interleukin-1 receptor antagonist used for the reduction in signs/symptoms and slowing the progression of structural damage in moderate to severe rheumatoid arthritis, in patients aged 18 years or older who have failed 1 or more disease modifying antirheumatic drugs (DMARDs). Anakinra is also used for treating patients with Neonatal-Onset Multisystem Inflammatory Disease (NOMID).
Dosage: The recommended dose of Anakinra to treat patients with rheumatoid arthritis is 100 mg daily administered by subcutaneous injection. The dose should be administered at around the same time every day. The recommended starting dose of Anakinra is 1 to 2 mg/kg for NOMID patients. The dose can be individually adjusted to a maximum of 8 mg/kg daily to control active inflammation. Adjust doses in 0.5 to 1.0 mg/kg increments. Once daily administration is generally recommended, but the dose can be split into twice daily administrations. Each syringe is intended for a single use. A new syringe must be used for each dose.
Side Effects: The most commonly reported anakinra side effects are: Rheumatoid Arthritis (RA): abdominal pain, flu like-symptoms, injection site reaction, nausea, arthralgia worsening of rheumatoid arthritis, upper respiratory tract infection, headache, diarrhea and sinusitis. NOMID: nasopharyngitis headache, vomiting, arthralgia, pyrexia and injection site reaction.
Warnings and Precautions:
- Do not use anakinra injection in patients with known hypersensitivity to E coli-derived proteins, anakinra, or any components of the product.
- Therapy with kineret 100 mg injection should not be started in those patients who are with active infections.
- Use of anakinra 100 mg injection in combination with TNF blocking agents is not recommended.
- Neutrophil counts should be assessed before initiating kineret 100 mg, and while receiving this drug, monthly for 3 months, and thereafter quarterly for a period up to 12 month.