Description
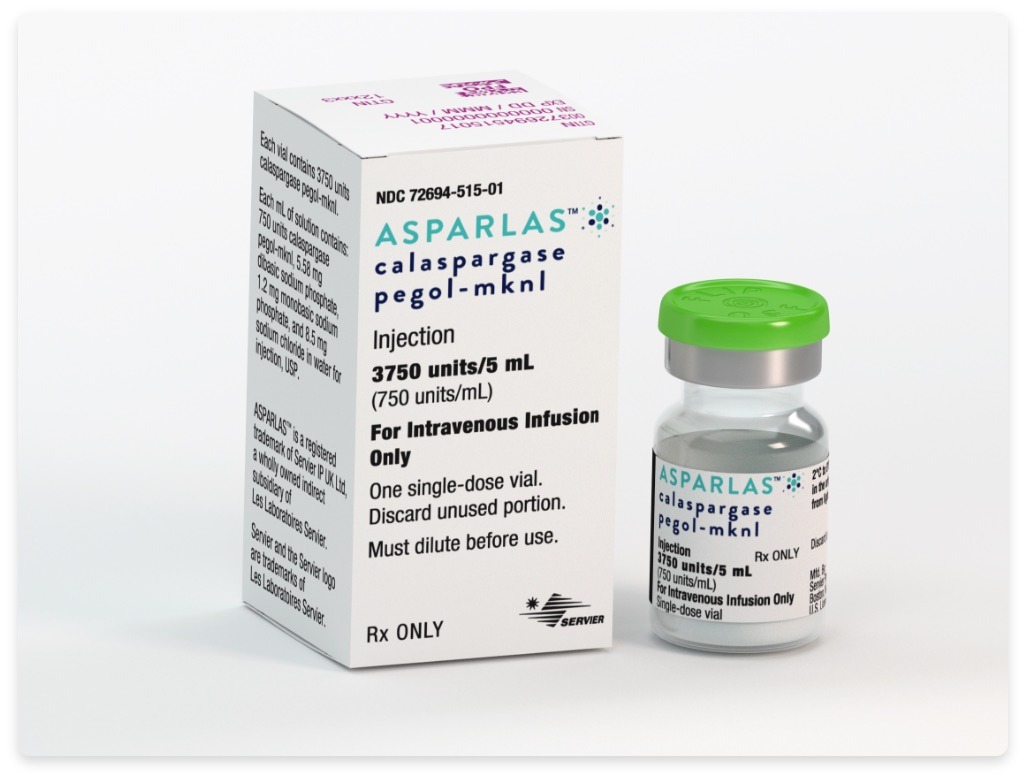
Uses of medication: Asparlas (Calaspargase pegol – mknl) is used as a component of a multi-agent chemotherapeutic regimen for the treatment of ALL (Acute Lymphoblastic Leukemia) in the young adult (aged 1 month to 21 years) and pediatric patients.
Dosage: The recommended asparlas dose is 2,500 units/m2, which should be administered intravenously not more frequently than every 3 weeks.
Treatment Reactions: The most commonly reported asparlas side effects include:
- Elevated transaminase
- Bilirubin increased
- Pancreatitis
- Abnormal clotting studies
Warnings and precautions
- The asparlas uses should be avoided in patients with the history of serious hypersensitivity reactions, including anaphylaxis, to pegylated L-asparaginase therapy.
- Patients receiving treatment with calaspargase 3750 injection should seek medical assistance in case they experience excessive thirst or any increase in the frequency of urination.
- Patients with asparlas 3750 injection should contact their doctor promptly if jaundice, severe nausea or vomiting, or easy bruising or bleeding occurs.
- Women with childbearing potential and receiving asparlas injection should avoid becoming pregnant.
- Due to the potential for adverse reactions in a breastfed baby, lactating women should avoid breastfeeding while on calaspargase pegol injection and for 3 months after the final dose.